INTRODUCTION OF FREEZING AND THAWING APPLICATION FOR MAMMALIAN CELLS(large volume)
With the development of cell processing medicines and regenerative products, there is an increasing demand for the storage of the large volume frozen cells and their use after thawing, instead of the conventional cryovial size( 1 to 2 mL). Thawing a large volume of frozen cells has the following issues:
▼
Labor saving and Automation
▼
Prevention of contamination
▼
Uniformity and Reproducibility
▼
Standardization of operation (gloval standard)
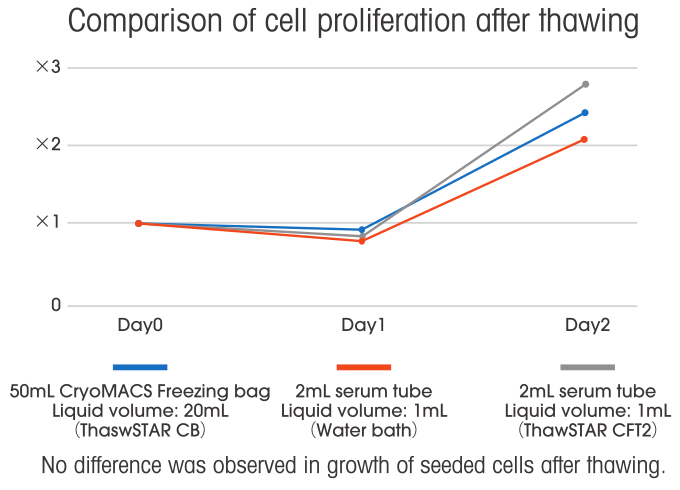
These issues can be overcome with CryoMACS Freezing Bags and ThawSTAR CB. However, there is another issue which is a decrease in cell viability when thawing a large volume of frozen cells. Therefore, with the cooperation of Dr. Shugo Tohyama at Keio University school of Medicine, we measured cell viability when using CryoMACS Freezing Bags and ThawSTAR CB.
Purpose of Research
Cryopreserving a large volume of human cells
Issue
When cryopreserving a large volume of human iPS cells, handling a large number of cryovials is necessary, and as a result, the freezing and thawing process takes a lot of time, and the viability decreases.
Resolving the issue
There is a possibility that using both of CryoMACS Freezing Bags and ThawSTAR CB solves the issue.

Researcher
Associate Professor, Keio University school of Medicine
Dr. Shugi Tohyama
main research
◉Cell production using metabolic properties
◉Regenerative medicine using human iPS cells
▼Experiment example▼
Cells used
Human iPS cells
State
28 days cryopreservating
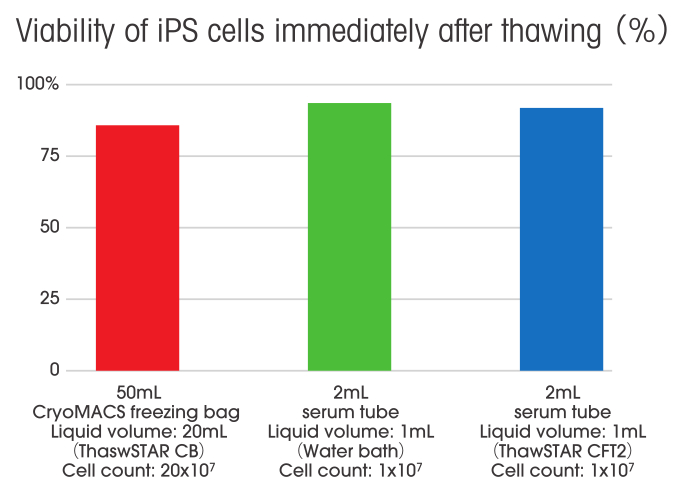
post-thaw viability
85%
Volume
20mL cell suspension(in 50mL freezing bag)
Frozen cell count
2×10⁸(1×10⁷cells/mL×20mL)
Thawing time
About 8 minutes
(from the start of thawing to the cell collection)
Time required for thawing
(Time to cell seeding*)
- 2mL vial water bath
2min 30sec(3min 35sec*) - 2mL vial and ThawSTAR CFT2
3min 14sec (4min 6sec*) - 50mL bag and ThawSTAR CB
3min 51sec (8min 13sec*)
The amount of liquid in the bag is large, so it takes time to process.
OPTIMAL FREEZING AND THAWING OF CELLS CAN BE ACHIEVED!
You can get them at Waken B Tech Co., Ltd.
Product introduction
ThawSTAR CB
frozen cells thawing system(for freezing bags)

※ThawSTAR CB is not a medical device stipulated by the Pharmaceutical and Medical Devices law in Japan.
- Compatible with 50mL – 750mL CryoMACS freezing bags
(Capable of thawing multiple sizes with one unit) - Water-free(Reduced contamination risk)
- Automatically thawed(No difference due to people’s techniques)
- Contributes to the reproducibility of the thawing state
(Remaining ice prevents excess temperature rise) - Easy operation(Intuitive operation eith touch screen)
- Thawing temperature log can be collected
(Temperature measurement at pre-configured points of the bag)
- Lineup from 50 to 1,000mL
- Certified Medical device in Japan⟨Class Ⅱ⟩(Optimal for medical purposes)
Medical device certification number:223ACBZX00071000 Manufacturer:Miltenyi Biotech Co., Ltd. - Can be used for research, medicine and manufacturing
(Suitable for scaling-up storage) - Multiple tube lines
(Various connectors and multiple ports) - Worldwide availability