Why should we choose peracetic acid to decontaminate lab equipment?
Introduction
In order to reduce contamination risks or aseptically manufacture products in laboratories or CPCs, periodic decontamination and cleaning are needed to strictly conduct microbial control.
There are many methods for decontamination such as formaldehyde fumigation, chlorine dioxide gas decontamination and so on, but we recommend using peracetic acid.
Peracetic acid is widely used for various fields in the world, for example medical device decontamination and food processing. Also, it is said that peracetic acid is much safer than formaldehyde because it is not carcinogenic to humans. In this web page, we introduce the reasons why we should choose peracetic acid to decontaminate lab equipment, comparing other decontamination agents.
(Please note that formaldehyde or chlorine dioxide is usually used to decontaminate safety cabinets.)
Peracetic acid and hydrogen peroxide
【Peracetic acid】
Peracetic acid is effective against almost all microorganisms including spore-forming bacteria and viruses. The chemical formula of peracetic acid is “CH3COOOH” and it typically exists as equilibrium mixture of acetic acid, hydrogen peroxide, and peracetic acid. (Please refer to the following image)
Peracetic acid is not specified as deleterious substances because they contain low concentration of hydrogen peroxide. (The regulation varies depending on the country. Please refer to the local laws and regulations in your country.)
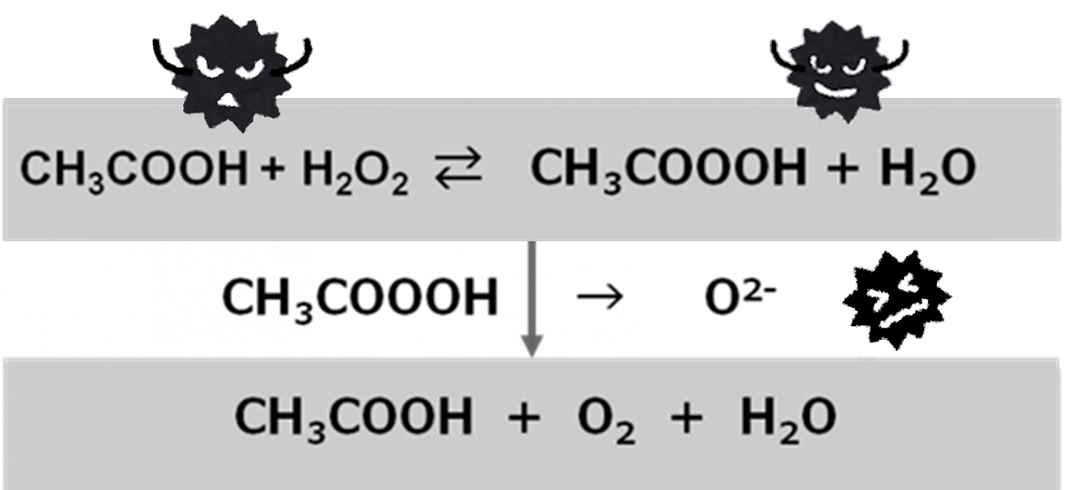
O2- radical is released when peracetic acid is degraded to acetic acid and it has a wide effect on various microbials including spore-forming bacteria. Peracetic acid turns to non-harmful substances like acetic acid, oxygen and water after it is degraded.
【Hydrogen peroxide】
Hydrogen peroxide is also effective against almost all microorganisms including spore-forming bacteria. Hydrogen peroxide gas decontamination or hydrogen peroxide gas plasma decontamination is mainly used for lab equipment decontamination. Solutions containing hydrogen peroxide at certain concentration or more are specified as harmful substances. Hydrogen peroxide solution used for gas fumigation usually contains high concentration of hydrogen peroxide, operators must handle it with much care. Hydroxy radical is released when hydrogen peroxide is degraded.
H₂O₂ → H₂ + ・OH
Hydroxy radical attacks microorganisms and is effective against microorganisms.
Peracetic acid dry fog decontamination by FOGACT

FOGACT is a compact device that can be used for lab equipment decontamination.
FOGACT generates dry-fog of peracetic acid (Spor-Klenz RTU) and the dry-fog decontaminates your equipment thoroughly.
Dry-fog means fog formed by particles of 6µm or less in diameter.
Since it doesn’t make lab equipmment damp due to its extremely small particles, operators do not have to wipe out residues after decontamination with FOGACT.
It also contributes to reducing risks of contamination and secondary contamination.
FOGACT is portable and can be placed in an incubator and a safety cabinet due to its compact size and light weight.
FOGACT can be easily used because it automatically calculates decontamination time based on temperature and humidity. The decontamination process can be started just pressing a few buttons.
Biological indicators are also available. Operators can validate peracetic acid decontamination process by using the BI.
Comparison between Peracetic Acid Dry Fog Decontamination, Hydrogen Peroxide Gas/Gas Plasma Decontamination and Folmaldehyde fumigation
| Item | Peracetic acid dry-fog decontamination | Hydrogen peroxide gas plasma decontamination | Formaldehyde fumigation |
|---|---|---|---|
| Safety | 〇 | △ (High concentration solution could be specified as deleterious substances) |
× (Carcinogenic) |
| Efficacy | 〇 | 〇 | 〇 |
| Corrosiveness | Strong | Strong | Mild |
| After treatment | Ventilation (Wiping out could be sometimes needed) | Neutralization and wiping out | Neutralization, ventilation and wiping out |
| Advantages | Easy to handle. | Decontamination time could be shorter than the others. | Corrosiveness is lower than the other 2 methods. Costs are low. |
| Disadvantages | Odor like acetic acid. Decontamination time is relatively longer. Corrosive. |
Deleterious substances. Corrosive. Harmful (High concentration solution) | Carcinogenic. Decontamination time is relatively longer. Residual property |
